GMP biopharmaceutical purified water
GMP (Good Manufacturing Practice) is an abbreviation for Good Manufacturing Practice, which ensures that the entire process of drug production, from raw material procurement, production, quality control to storage and distribution, meets regulatory requirements. In GMP standards, purified water is an important raw material in the drug production process, and its quality directly affects the safety and effectiveness of the drug. Therefore, purified water that meets GMP standards must comply with a series of strict quality requirements and production regulations
Product details
1、 Functions of injection pharmaceutical purified water equipment
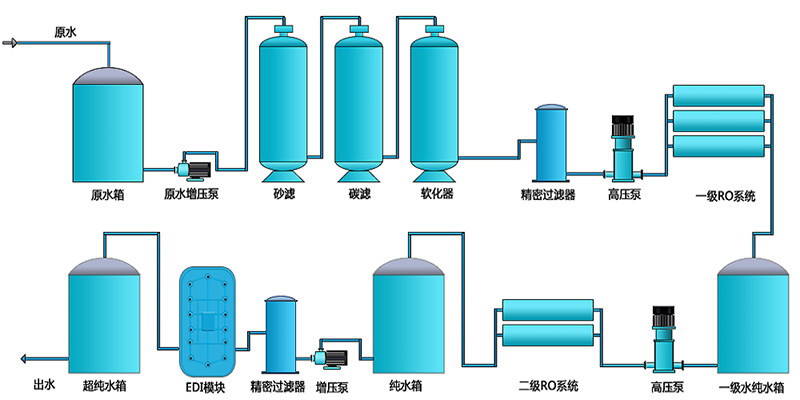
1. The raw water tank, intermediate water storage tank, RO membrane, EDI, and purified water storage tank can be cleaned online;
2. Whole machine horizontal block design, automated control;
3. The preparation system terminal adopts a qualified water dual circulation water supply mode to enter the purified water storage tank. Unqualified water circulates back to the intermediate water storage tank. When the purified water storage tank is full, it automatically switches to the self circulation state of each module to ensure that there is no dead water in the system;
4. The control system adopts PLC automatic control, which meets the verification requirements of GAMP5 guidelines and the electronic record and signature requirements of 21CFR Part 11;
5. Equipped with online monitoring, timed printing, and water quality exceeding alarm functions, we provide protection for your production water supply.
2、 Characteristics of injection pharmaceutical purified water equipment
1. The structural design is simple, reliable, and easy to disassemble. The design of the actuator should use standardized, universal, and systematic components as much as possible.
2. The entire system of the ultra pure water treatment equipment is also made of all stainless steel materials, which are smooth, flat, without dead corners, easy to clean and sterilize, and corrosion-resistant to prevent rusting.
3. Directly using tap water to produce sterile ultrapure water can completely replace distilled water and double distilled water.
4. We use high-quality components such as imported pumps and reverse osmosis membranes.
5. Fully automatic operating system, efficient automatic flushing
6. Using imported instruments, it can accurately and continuously analyze and display water quality.
7. Applied to pure water for pharmaceutical industry, medical infusion preparations, and purification of medical sterile water
Technical Parameter
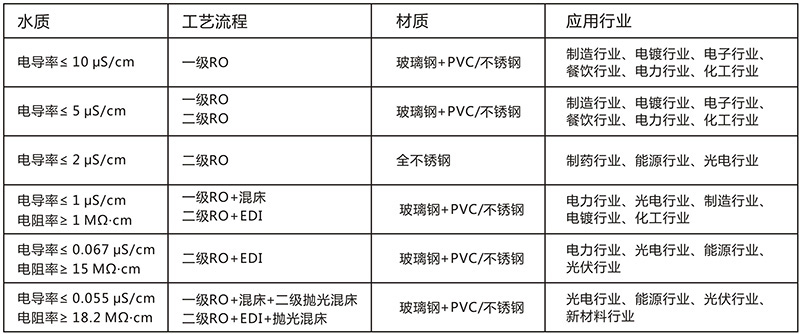
Scope of application
Technological process